
Alpha particle skin#
A health hazard occurs when material contaminated with α-emitting radionuclides is eaten or inhaled, or otherwise absorbed inside the body, so that organs and tissues more sensitive than skin are exposed to α radiation.

1 External radiation by α particles presents no direct health hazard because even the most energetic are stopped by the epidermal layer of skin and rarely reach more sensitive layers. α particles thus have a mass of about 4 amu (6.642×10 −4 g) each and a positive charge of 2. Controlled measurement of alpha (α), beta (β), and gamma (γ) radiationĪlpha radiation has been identified as helium nuclei that have been stripped of their planetary electrons, and each consists of two protons and two neutrons. Near the very end of the travel, the specific ionization decreases to zero as the particle acquires two electrons and becomes a neutral atom.įIGURE 16-2. For this reason, the specific ionization increases near the end of the alpha particle's travel. As the alpha particle gives up its energy, it slows and therefore spends more time in the vicinity of atoms. In air, the specific ionization may be ∼10,000 ion pairs per centimeter or more. It should be clear then that a typical alpha particle, with perhaps 5 MeV of energy, will cause a large amount of ionization and it is safe to say that alpha particles have a high specific ionization. Only ∼34 eV of energy is required to produce an ionizing event in a gas such as air. The specific ionization of alpha particles is, of course, dependent on the energy of the radiation. The number of ion pairs created per unit length of travel is called the specific ionization. In these situations, the electron may be imagined as being “ripped” from its orbit as the alpha particle passes nearby. The closer an alpha particle passes near an electron the stronger the force and the higher the probability an ionizing event will occur. In some cases, this force is not sufficient to separate the electron from the atom, but the electron is raised to a higher energy state and the atom is said to be “excited.” In other cases, the attractive force is sufficient to remove the electron from the atom (ionization). Since the particle is positively charged, it exerts an attractive force on the oppositively charged electron. These interactions are with the loosely bound, outer electrons of the atoms in the material and should not be considered collisions. The alpha particle has a high electrical charge but a low velocity due to its large mass, and interactions are frequent. The major energy-loss mechanisms are electronic excitation and ionization. Poston Sr., in Encyclopedia of Physical Science and Technology (Third Edition), 2003 III.A Alpha RadiationĪs charged particles, such as alpha particles, move through material, energy is transferred from the radiation to the atoms or molecules that make up the material. The chapter also presents the formulas for deriving the range of alpha particles in liquids, solids, and air.

Depending on the absorbing material, the excited atoms or molecules of the material immediately fall back to a lower energy state or ground state by dissipating the absorbed energy as photons of visible light. Rather, the atoms or molecules of a given material absorb a portion of the alpha-particle energy and become elevated to a higher energy state. However, electron excitation occurs when the alpha particle fails to impart sufficient energy to an atomic electron for it to be ejected from the atom. The high mass and charge of an alpha particle, relative to other forms of nuclear radiation, give it greater ionization power but a poorer ability to penetrate matter. Alpha particles as well as other types of charged particles dissipate their energy during these collisions mainly by two mechanisms: ionization and electron excitation.
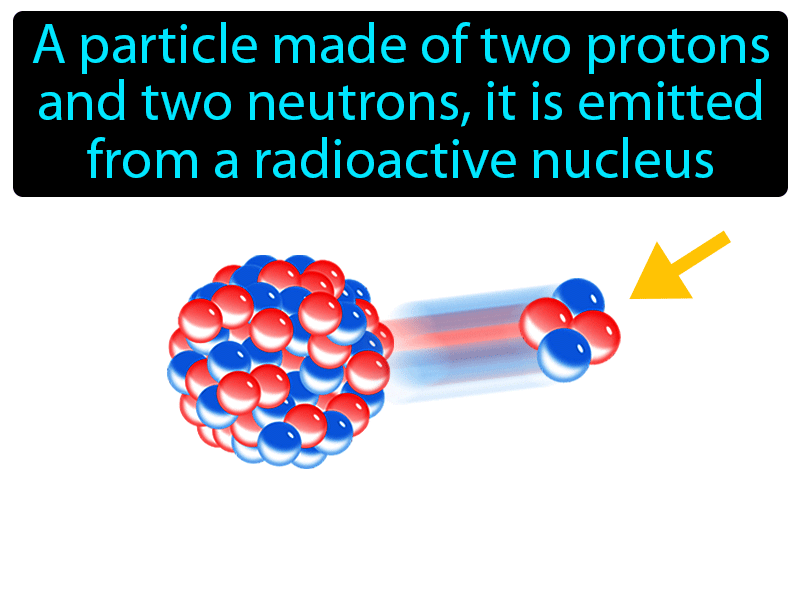
The two neutrons of an alpha particle give it additional mass that further facilitates ionization by coulombic interaction or even direct collision of the alpha particle with atomic electrons. During the process of nuclear decay, the liberated energy (decay energy) is shared between the daughter nucleus and the alpha particle. An alpha particle, structurally equivalent to the nucleus of a helium atom, consists of two protons and two neutrons. This chapter discusses various aspects of alpha radiation, which is made up of alpha particles. L'Annunziata, in Radioactivity, 2007 Publisher Summary


 0 kommentar(er)
0 kommentar(er)
